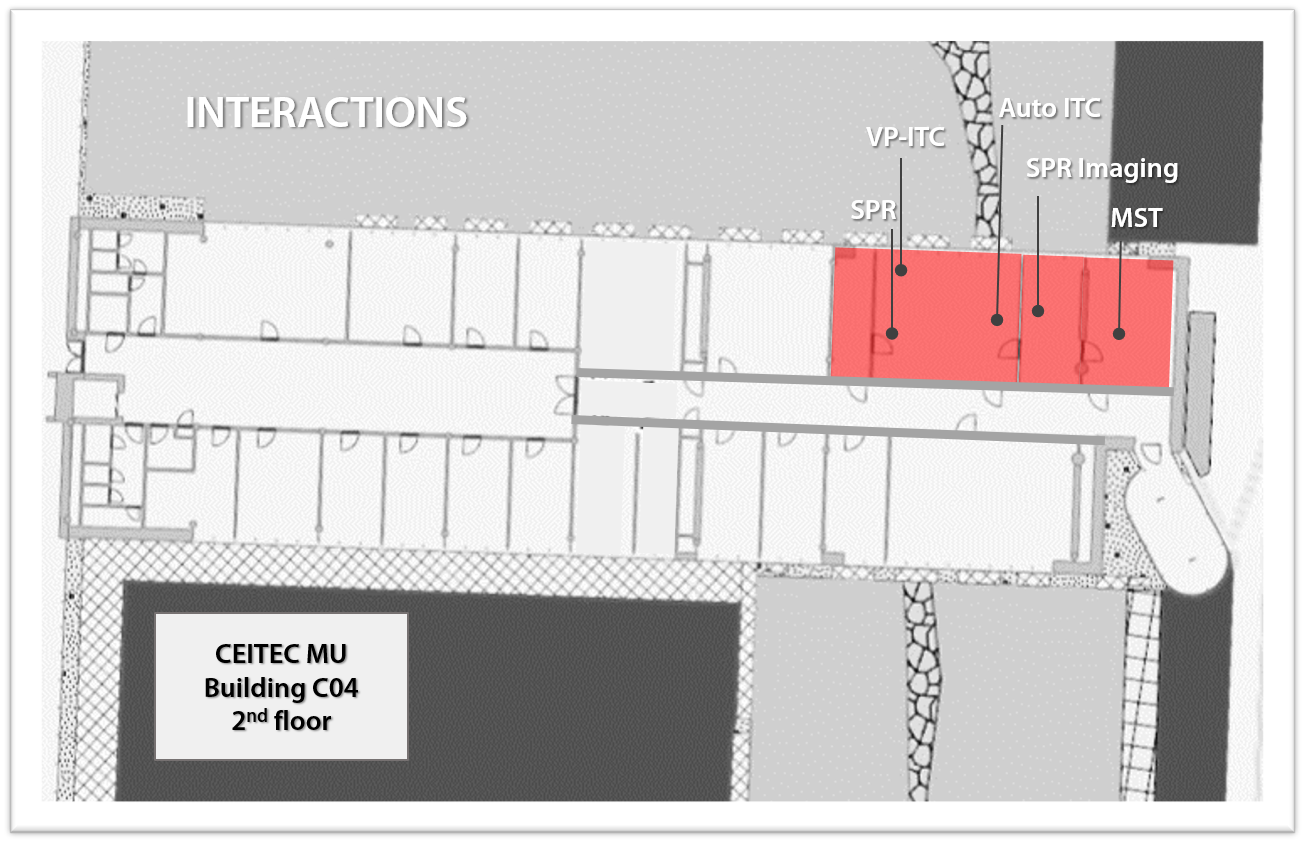
Equipment - Interactions
The interaction of biomolecules determines all processes in living organisms. Over the years, a broad range of techniques has been developed in order to analyze interactions of proteins, nucleic acids and small molecules. Utilizing different principles, the methods allow to estimate thermodynamic (ITC)and kinetic (SPR) parameters. While some techniques observe the interaction of intact particles in a solution (ITC), others may require specific labeling (MST) or immobilization onto a surface (SPR, BLI). AUC not only helps characterize the samples but can be used also for biomolecular interaction or self- and hetero-association systems studies. The suitability for a particular type of the molecule also varies, with some methods being more successful in small molecule binding experiments than others.
Interactions can be studied not only by typical technique but also techniques that are used for characterization - stability techniques proved to be very helpful in interaction studies. This is based on persumption of more stable complex being created upon binding thus the (thermal) stability increases. This allows to use data from nanoDSF and DSC not only for characterization but also for confirmation of interactions.

Mass Photometry : TwoMP with MassFluidix
Mass photometry is a cutting-edge analytical technique that allows researchers to measure the mass of individual molecules in solution without requiring labels or extensive sample preparation. Our TwoMP instrument is available also with the lastest inovation - the MassFluidix High Concentration microfluidic system - which enables measurement of low-affinity biomolecular interactions through rapid dilution.
Analytical ultracentrifugation: Optima and ProteomeLab XL-I
Beckman Coulter ProteomeLab XL-I and Optima analytical ultracentrifuge is equipped with absorbance (wavelength range 190 – 800 nm) and interference optics and can be used for both sedimentation velocity and sedimentation equilibrium experiments. Analytical ultracentrifugation is applicable for proteins, nucleic acids, lipids, polysaccharides, viruses and nanoparticles. It has a broad applicability including determination of sample homogeneity, oligomeric state of proteins (or molecular weight, respectively) and can be used to assess aggregation of sample and to study biomolecular interactions of self- and hetero-association systems (determination of stoichiometry and affinity).
PROFILE CARD (OPTIMA) PROFILE CARD (PROTEOMELAB) SERVICE MODE
Calorimetry: PEAQ-ITC Automated
Fully automated isothermal calorimeter PEAQ-ITC Automated with ultrasmall working cell (200 µl). ITC method is used for characterization of biomolecular interactions of small molecules, proteins, antibodies, nucleic acids, lipids and others. Enzyme kinetics, biological activity or the effect of molecular structure changes on binding mechanism can be also assessed. Complete thermodynamic profile of the molecular interaction in a single experiment (stoichiometry, Ka, ∆H and ∆S values).
VP-ITC is isothermal calorimeter with 1400 µl volume of working cell. ITC method is used for characterization of biomolecular interactions of small molecules, proteins, antibodies, nucleic acids, lipids and others. Complete thermodynamic profile of the molecular interaction can be obtained in a single experiment (stoichiometry, Ka, ∆H and ∆S).
Microscale thermophoresis: Monolith NT.115
The Monolith system measure equilibrium binding constants for a variety of molecules – thus allows to measure wide range of interactions from ion fragment binding up to interactions of large complexes (liposomes and ribosomes).

Microscale thermophoresis: Monolith NT.115 Pico
The Monolith systems measure equilibrium binding constants for a variety of molecules – thus allows to measure wide range of interactions from ion fragment binding up to interactions of large complexes (liposomes and ribosomes).
Biolayer interferometry : Octet RED96e
Biosensor system Octet utilizes BLI technology (biolayer interferometry) to detect the interaction parameters (kinetics, affinity) on a sensor chip. Octet RED96e enables simultaneous measurement of 8 channels ensuring high throughput. System is suitable especially for protein-protein or protein-nucleic acid interactions, however other binding systems can be evaluated as well.
Surface plasmon resonance: BiaCore S200
Biacore S200 is a successor of T200 model with higher sensitivity. This system monitors real-time, label-free interactions ranging from ions to viruses. The system offers a powerful, versatile solution for obtaining the highest quality kinetic, affinity, concentration, specificity, selectivity, and thermodynamic data and can be used for multiple applications, from early research to drug discovery and development. In the arrangement, one of the interactants is immobilized on the sensor chip surface, while the other is passed over that surface in solution.
Fluorescence Activated Cell Sorting (FACS): CytoFlex SRT
The CytoFLEX SRT is a compact cell sorter designed to handle a wide range of samples commonly processed in a core facility. From bacteria to tumor cells and everything in between, the SRT provides the versatility needed to support the diverse research community.
Surface plasmon resonance: Imaging system
Multichannel (36) SPR biosensor system for affinity studies of biomolecules, flow-through design, parallel analysis of samples.